Iranian-made vaccine against Omicron begins clinical trial
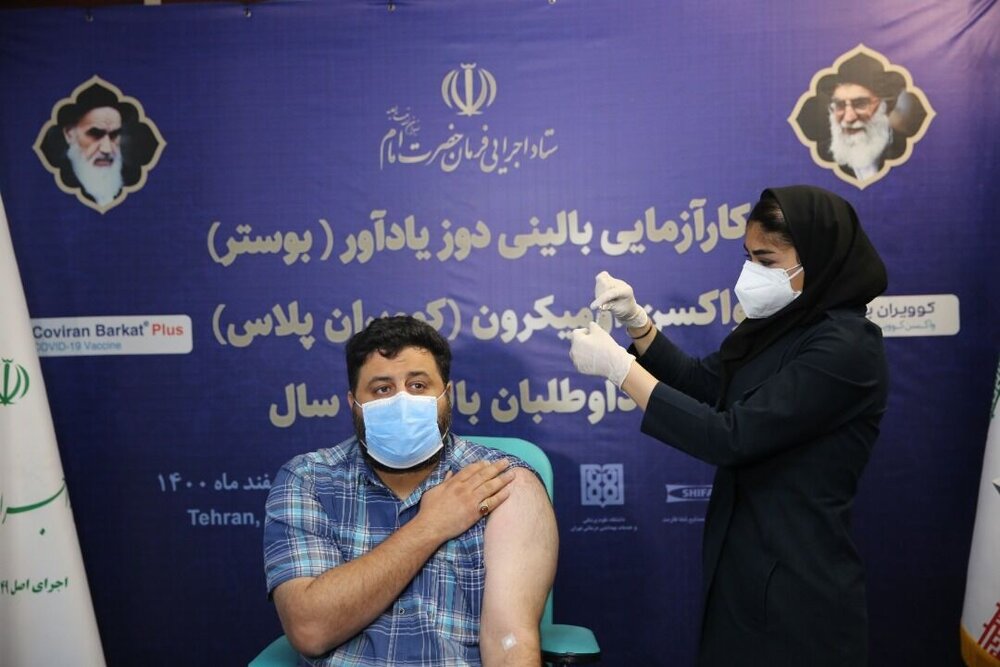
TEHRAN – COVIRAN plus, the first homegrown vaccine against Omicron strain, started the human trial by being injected into 210 volunteers, Minoo Mohraz, a member of the National Scientific Committee of COVID-19, has stated.
In this study, three groups of 70 people will be vaccinated, she said, adding that COVIRAN Plus will be administrated to 140 volunteers who have received their first two doses of COVIRAN or Sinopharm, and 70 people will be injected with the COVIRAN vaccine.
The results will be handed over to the Ministry of Health by the next two weeks, she noted.
“Like all other vaccine manufacturing companies around the world, COVIRAN started development to be effective against the new variant and it led to the production of COVIRAN plus so that some 60 million doses of the COVIRAN have so far been produced.”
Made by researchers at the Headquarters for Executing the Order of the Imam, COVIRAN Barkat was unveiled on December 29, 2020, and received the license for public use on June 14.
COVIRAN is the first vaccine in West Asia that is in the process of global registration.
Iran is the sixth country in the world and the first country in West Asia to gain the ability to produce the Coronavirus vaccine.
More effective than world-known vaccines
According to a new study, the effectiveness of the Iranian-made COVIRAN Barkat vaccine in fighting the coronavirus has been more than foreign rivals, namely Sinopharm, AstraZeneca, and Sputnik.
The study was performed on 1.8 million people in Fars province from the beginning of the vaccination process till October 2021, which considered four vaccines of Sinopharm, AstraZeneca, COVIRAN, and Sputnik.
COVIRAN vaccine was 87 percent effective in protecting against coronavirus infection and 86 percent effective against Covid-related hospitalization, compared with 84 percent and 82 percent, respectively for AstraZeneca; Sinopharm came in third with 80 percent and 72 percent, respectively.
FB/MG
Leave a Comment